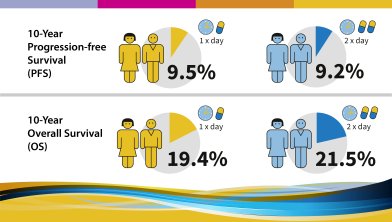
Ten-year progression-free and overall survival in patients with unresectable or metastatic gastrointestinal stromal tumours (GIST). Long-term analysis of the EORTC, ISG, AGITG intergroup Phase III randomized trial on imatinib at two dose levels.
HOW MANYpatients participated? |
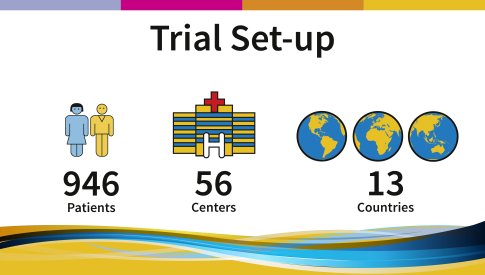
A total of 946 patients at 56 centers in 13 countries in Europe and Australia/New Zealand/Singapore took part in this trial. The study was open for enrollment from February 2001 till February 2002 and constitutes one of the first large trials of Imatinib (Glivec®/Gleevec®) in GIST. |
STUDY DESIGN:What does the study look like? |
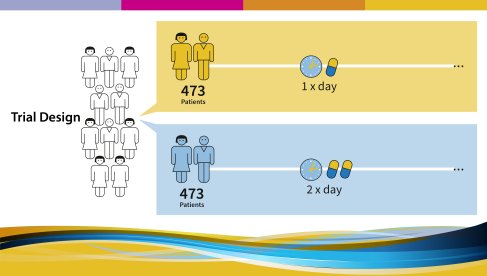
All participating patients were randomly divided into two groups: 473 patients were treated with Imatinib 400mg once daily, 473 patients were treated with imatinib 400 mg twice daily (total dose/day: 800 mg).
The two groups were evenly balanced on the basis of demographic and clinical characteristics of the patients. The majority of patients (72%) had received prior surgery (85 %), chemotherapy (33 %) and/or radiotherapy (7 %) for advanced GIST. |
RESULTSof the study? |
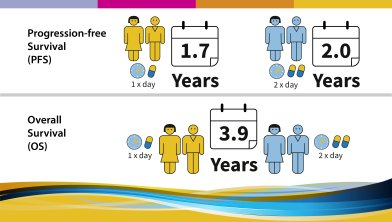
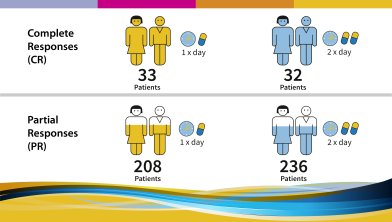
The trial aimed to answer several questions:
The following results were observed and published in 2017:
|
CONCLUSION |
This trial was initiated not long after imatinib became available for the treatment of GIST approximately 15 years ago. Little was known about GIST and the activity and potential of imatinib at the time. These 10-year data show today that there is no difference in outcome (progression-free survival or overall survival) between the two doses of Imatinib. However, it confirms that patients with KIT exon 9 mutation benefit from starting on the higher dose of imatinib and that a higher dose is also beneficial for patients progressing under 400 mg imatinib once daily. It has to be noted though, that given the limited knowledge at initiation of this study, mutational analysis wasn’t carried out for all patients. Study investigators resume that the prognosis of advanced GIST patients starting imatinib today might be better overall due to improved and deeper knowledge about GIST. The publication of this study can be viewed here. |
SHAREyour experience |
Were you one of the patients on this trial? If you want to share your experience with others, please send us an e-mail to: This email address is being protected from spambots. You need JavaScript enabled to view it. Note that your experience would be helpful for other patients and patient organisations. Your name and details will not be published or shared without your explicit permission in writing. |
Disclaimer: This is a patient-friendly summary of the clinical trial which has been provided for informational purposes only. Patients should consult their physician about any clinical trial opportunity.