Selinexor in Advanced Liposarcoma (SEAL)
| Agents: | Selinexor |
| Phase | II/III |
| Status | Closed, active |
| Sponsor | Karyopharm Therapeutics Inc |
Further information: https://clinicaltrials.gov/ct2/show/NCT02606461
This is a randomized, multicenter, double-blind, placebo-controlled, Phase 2-3 study of patients diagnosed with advanced unresectable dedifferentiated liposarcoma (DDLS)
WHO is the trial for? |
|
WHAT is the key question that this trial is attempting to answer? |
This study aims to investigate a drug called selinexor, a first-in-class, oral selective inhibitor of nuclear export (SINE). This trial tries to find out whether taking selinexor after treatment with at least two up to five prior therapies is effective and safe for patients who progressed on prior therapy. |
WHY patients might want to participate? |
This clinical trial offers patients an opportunity to access a new therapy strategy. This trial will further support the research in sarcomas and potentially help other patients with this disease. The trial might or might not have benefit in your individual case. For more about the importance and benefits of joining clinical trials, please click here. |
WHEN will the trial be open? |
Phase III of this study is closed for recruitment, but active. |
WHERE is the trial available? |
The trial is being conducted in several study centers in Canada, France, Germany, Israel, Italy, Spain, Sweden, the UK and the US. For further information please check here: https://clinicaltrials.gov/ct2/show/NCT02606461 Study contact:
|
STUDY DESIGN: What does the study look like? |
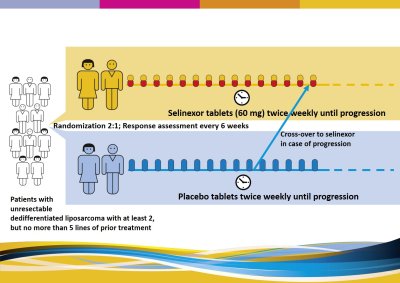
This study has two phases: phase II and phase III. Both phases contain two study arms (groups): all participating patients will be randomly divided (by a computer) in two groups. In phase II of the study, half of the participanty will received selinexor, the other half received placebo. In phase III, two thirds of participants will receive selinexor, one third wil receive placebo. Patients, who might progress on placebo can receive/cross-over to the selinexor arm. Phase II of this study is completed, phase III is ongoing. Design of phase III of this study:
|
HOW do I get more information? |
Patient organisations supporting sarcoma and/or GIST patients in your country may offer additional information about the trial, current recruitment status, and key contacts. Click here for a list of patient organisations serving GIST and/or sarcoma patients. If there are no such organisation in your country, please email us for more information: このメールアドレスはスパムボットから保護されています。閲覧するにはJavaScriptを有効にする必要があります。 |
CONNECT with other patients on this trial |
If you want to connect with other patients considering or participating in this trial, you can find them here. |
SHARE your experience |
You want to share your experience on this trial? Send us an e-mail to: このメールアドレスはスパムボットから保護されています。閲覧するにはJavaScriptを有効にする必要があります。 Note that your experience would be helpful for other patients and patient organisations. |
RESULTS of the study |
Results for phase II of this study are available here (company information). |
Disclaimer: This is a patient-friendly summary of the clinical trial which has been provided for informational purposes only. Patients should consult their physician about any clinical trial opportunity.