Randomized trial of Crenolanib in patients with D842V mutated GIST
| Trial name | CrenoGIST |
| Agents: | Crenolanib vs. placebo |
| Phase | III |
| Status | Closed, active |
| Sponsor | Arog Pharmaceuticals, Inc. |
Further information: https://clinicaltrials.gov/ct2/show/NCT02847429?term=crenolanib+Gist&rank=2
WHO is the trial for? |
|
WHAT is the key question that this trial is attempting to answer? |
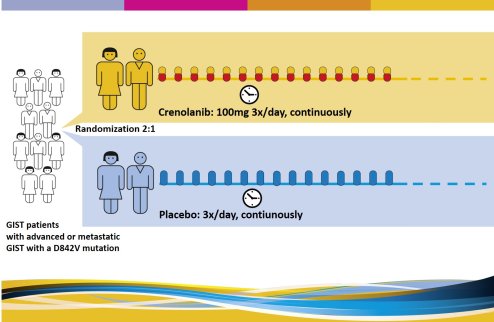
This study assesses the safety and efficacy of crenolanib in patients with advanced or metastatic GIST with a D842V mutation in the PDGFRA gene. |
WHY patients might want to participate? |
This clinical trial offers patients an opportunity to access a new therapy strategy and will further support the research in GIST and potentially help other patients with this disease. The trial might or might not have benefit in your individual case. For more about the importance and benefits of joining clinical trials, please click here. |
WHEN will the trial be open? |
The study is closed, but active. |
WHERE is the trial available? |
The trial is being conducted in several study centers in the US, France, Norway, Germany, Italy, Spain and Poland. For further information please check here. Information Contact: This email address is being protected from spambots. You need JavaScript enabled to view it. |
STUDY DESIGN: What does the study look like? |
There are two study arms (groups): all participating patients will be randomly divided (by a computer) in two groups. |
HOW do I get more information? |
Patient organisations supporting GIST and/or sarcoma patients in your country may offer additional information about the trial, current recruitment status, and key contacts. Click here for a list of patient organisations serving GIST and/or sarcoma patients. If there are no such organisation in your country, please email us for more information: This email address is being protected from spambots. You need JavaScript enabled to view it. |
CONNECT with other patients on this trial |
If you want to connect with other patients considering or participating in this trial, you can find them here: List of organisations worldwide. |
SHARE your experience |
You want to share your experience on this trial? Send us an e-mail to: This email address is being protected from spambots. You need JavaScript enabled to view it. Note that your experience would be helpful for other patients and patient organisations. |
RESULTS of the study |
No results are available at this time. Future results will be linked here. |
Disclaimer: This is a patient-friendly summary of the clinical trial which has been provided for informational purposes only. Patients should consult their physician about any clinical trial opportunity.