Maintenance therapy with Cabozantinib in High Grade Uterine Sarcoma (HGUtS)
| Agents: | Cabozantinib |
| Phase | II |
| Status | Open, recruiting |
| Sponsor | EORTC |
Further information: https://clinicaltrials.gov/ct/show/NCT01979393 or http://www.eortc.org/research_field/clinical-detail/62113/
A phase II study evaluating the role of maintenance therapy with Cabozantinib in High Grade Uterine Sarcoma (HGUtS) after stabilization or response to chemotherapy
WHO is the trial for? |
|
WHAT is the key question that this trial is attempting to answer? |
This study aims to investigate a drug called Cabozantinib which belongs to a family of drugs that have effects on tumour growth, blood supply, invasion and spread.
|
WHY patients might want to participate? |
This clinical trial offers patients an opportunity to access a new therapy strategy. This trial will further support the research in sarcomas and potentially help other patients with this disease. The trial might or might not have benefit in your individual case. For more about the importance and benefits of joining clinical trials, please click here. |
WHEN will the trial be open? |
The study is open (recruiting) for participation. |
WHERE is the trial available? |
The trial is available in several study centers in the France, Belgium, The Netherlands, Spain, Italy and the UK. For further information please check here: http://www.eortc.org/research_field/clinical-detail/62113/ or https://clinicaltrials.gov/ct/show/NCT01979393 Study contact:
|
STUDY DESIGN: What does the study look like? |
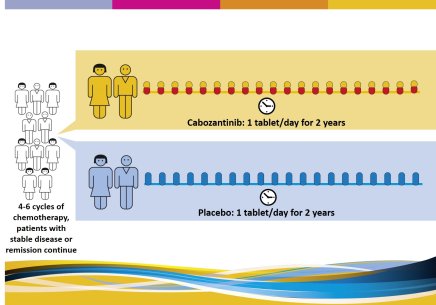
All participants will receive 4-6 cycles of standard chemotherapy. Then, there will be 2 study arms (groups): those patients with stabilization or response to the standard chemotherapy will be randomly divided (by a computer) in two groups and receive either cabozantinib or placebo.
|
HOW do I get more information? |
Patient organisations supporting sarcoma and/or GIST patients in your country may offer additional information about the trial, current recruitment status, and key contacts. Click here for a list of patient organisations serving GIST and/or sarcoma patients. If there are no such organisation in your country, please email us for more information: This email address is being protected from spambots. You need JavaScript enabled to view it. |
CONNECT with other patients on this trial |
If you want to connect with other patients considering or participating in this trial, you can find them here. |
SHARE your experience |
You want to share your experience on this trial? Send us an e-mail to: This email address is being protected from spambots. You need JavaScript enabled to view it. Note that your experience would be helpful for other patients and patient organisations. |
RESULTS of the study |
No results are available at this time. Future results will be linked here. |
Disclaimer: This is a patient-friendly summary of the clinical trial which has been provided for informational purposes only. Patients should consult their physician about any clinical trial opportunity.