Three years of imatinib (Gleevec, Glivec) is considered the standard duration of adjuvant (preventive) therapy for patients with operable GIST, but a high risk of relapse.
Yet, many patients are still at a high risk of GIST recurrence after completion of 3 years of adjuvant imatinib, and might benefit from further adjuvant imatinib therapy. The risk of GIST recurrence after 3 years of adjuvant imatinib can be estimated using prognostic factors, e.g. tumour mitotic count and tumour site. Patients with a high tumour mitotic count and GIST located at a nongastric site have the greatest risk.
| Trial name | SSG XXII: Three versus five years of adjuvant imatinib in patients with operable GIST |
| Agents |
Imatinib |
| Phase | III |
| Status | Open, recruiting |
| Sponsor |
Scandinavian Sarcoma Group |
Further information: https://www.clinicaltrials.gov/ct2/show/NCT02413736?term=adjuvant+imatinib&cond=GIST&rank=5
WHO is the trial for? |
Important: Patients with metastatic GIST or patients that had a relapse cannot take part in the study. Those patients usually receive imatinib lifelong or until disease progression. |
WHAT is the key question that this trial is attempting to answer? |
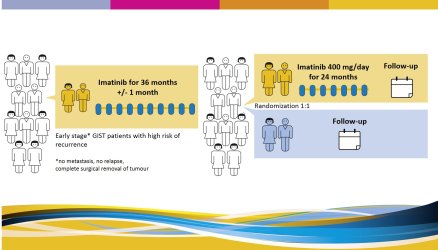
The study tries to answer the question if a prolonged adjuvant (preventive) therapy with imatinib for a total of 5 years will prevent some of the GISTs to recur as compared to patients who receive adjuvant imatinib for 3 years. It includes patients who have been diagnosed with GIST and have been treated with adjuvant imatinib for 3 years after surgery. |
WHY patients might want to participate? |
This clinical trial offers patients an opportunity to access a new therapy strategy and will further support the research in GIST and potentially help other patients with this disease. The trial might or might not have benefit in your individual case. For more about the importance and benefits of joining clinical trials, please click here. |
WHEN will the trial be open? |
The study is open (recruiting) |
WHERE is the trial available? |
The trial is available in various study centers across Europe: Sweden:
Finland:
Norway:
Denmark:
Spain:
UK:
Germany:
The Netherlands:
Austria:
Study coordinator: Scandinavian Sarcoma Group
|
STUDY DESIGN: What does the study look like? |
There will be 2 study arms (groups): all participating patients will be randomly divided (by a computer) in two groups.
|
HOW do I get more information? |
Patient organisations supporting GIST and/or sarcoma patients in your country may offer additional information about the trial, current recruitment status, and key contacts. Click here for a list of patient organisations serving GIST and/or sarcoma patients. If there are no such organisation in your country, please email us for more information: यह ईमेल पता spambots से संरक्षित किया जा रहा है. आप जावास्क्रिप्ट यह देखने के सक्षम होना चाहिए. |
RESULTS of the study |
No results are available at this time. Future results will be linked here. |
Disclaimer: This is a patient-friendly summary of the clinical trial which has been provided for informational purposes only. Patients should consult their physician about any clinical trial opportunity.